Landmark Study in Cell Unveils Comprehensive Protein Turnover Atlas Across Mouse Tissues
03
2025
In a groundbreaking study published in Cell, an international research team led by Prof. Yansheng Liu from Yale School of Medicine, in collaboration with Prof. Junmin Peng from St. Jude Children’s Research Hospital and Prof. Eugenio F. Fornasier from the University Medical Center Göttingen, has systematically mapped the protein turnover dynamics across multiple mouse tissues and brain regions. This work, titled Tissue-Specific Protein Turnover Atlas Reveals Dynamic Regulation of Proteostasis, provides unprecedented insights into how protein synthesis and degradation govern tissue-specific functions and homeostasis.
Key Findings and Methodology
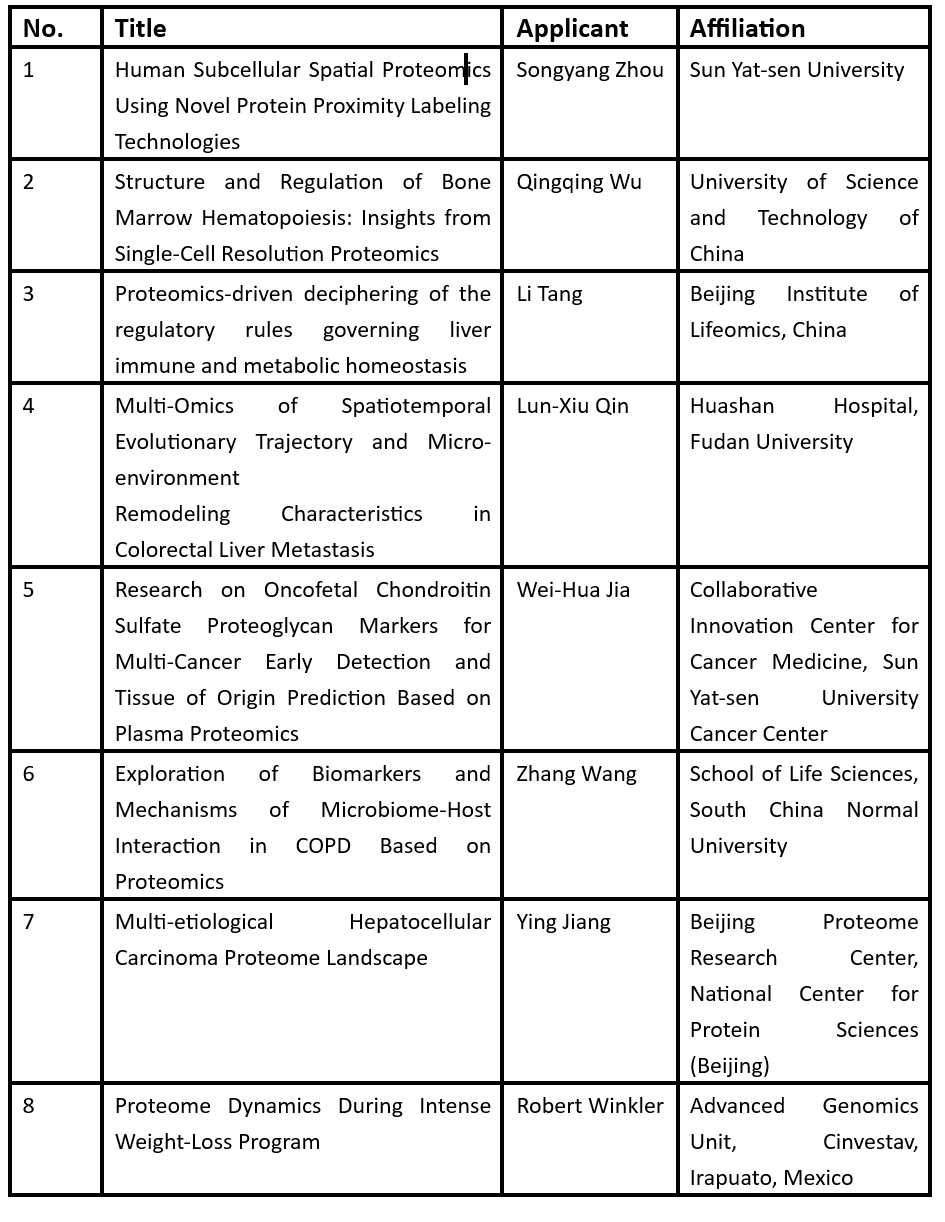
The study employed quantitative proteomics (DIA-MS and TMTpro labeling) combined with pSILAC (pulsed stable isotope labeling by amino acids in cell culture) to track over 11,000 proteins and 40,000 phosphorylation sites across eight tissues (heart, liver, spleen, lung, kidney, intestine, plasma) and nine brain regions (e.g., hippocampus, prefrontal cortex, substantia nigra). This dataset, accessible via the interactive platform Tissue-PPT, represents the most extensive resource on mammalian protein turnover to date.
1. Tissue-Specific Turnover Rates
Intestinal proteins exhibited the fastest turnover (average half-life: 3.27 days), while brain and cardiac proteins were the most stable (6.45 and 5.61 days, respectively). Notably, liver proteins showed rapid turnover despite low cell division rates, highlighting metabolic demand as a key regulator.
Protein-protein interaction (PPI) networks strongly correlated with turnover consistency: interacting proteins displayed synchronized degradation rates, suggesting functional co-regulation.
2. Phosphorylation Modulates Stability
Phosphorylation’s impact on protein stability varied by tissue. For example, phosphorylation of Tau and α-synuclein—critical in Alzheimer’s and Parkinson’s pathologies—prolonged their half-lives. Experimental dephosphorylation via PhosTAC accelerated Tau degradation, hinting at therapeutic strategies for neurodegenerative diseases.
3. Organelle-Specific Dynamics
Peroxisomal proteins demonstrated striking tissue variability. Accelerated turnover in the liver contrasted with delayed rates in the heart, aligning with their metabolic roles in lipid oxidation and reactive oxygen species detoxification.
Implications and Future Directions
This atlas not only deciphers the spatiotemporal regulation of proteostasis but also identifies potential therapeutic targets for diseases linked to protein dysregulation. The team plans to explore how pathological conditions alter turnover dynamics and develop therapies targeting protein degradation pathways.
Interactive Resource
The Tissue-PPT platform (https://yslproteomics.shinyapps.io/tissuePPT) enables researchers to explore tissue-specific protein expression and turnover data, fostering cross-disciplinary collaborations.
Original Paper
https://doi.org/10.1016/j.cell.2025.02.021

